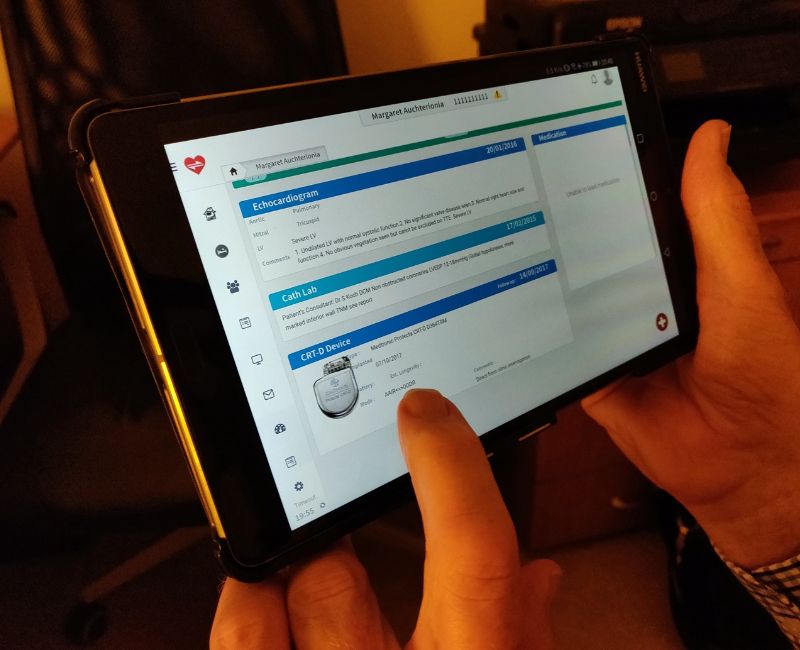
At the heart of EKORA® lies its Devices module. Used by all our customers across the NHS and beyond, the Devices module, designed by leading NHS Cardiologists, has been developed to offer the most intuitive platform to manage patients, devices, procedures and more alongside powerful search tools and data reporting.
This Modules in Focus explores how the Devices module addresses the needs of cardiac physiologists and cardiologists, providing a comprehensive, paperless solution that enhances clinical workflow, patient management, and data reporting.
Designed by practicing clinicians
Designed after extensive feedback and in-depth personal knowledge, the Devices module is tailored to meet the specific needs of cardiac physiologists and cardiologists:
- Seamless Clinical Workflow: Integrates smoothly into daily routines.
- Intuitive Interface: Ensures easy access to all necessary information.
- Comprehensive Design: Meets the various needs of a cardiology department.
A Complete Paperless Solution
EKORA® helps transition from paper-based records to a digital system:
- Centralised Management: Manage all device patient records in one system.
- Dashboard Overview: Provides a clear summary of all device patients.
- Detailed Records: Maintains in-depth records for each patient.
- Manual Document Integration: Allows adding scanned documents to reports or patient records.
In-Clinic and Remote Monitoring
EKORA® supports both in-clinic and remote monitoring:
- Detailed Monitoring Records: Keeps track of both remote and in-clinic check details.
- Data Flow Overview: Monitors data flow from remote and in-office uploads.
- Procedure Documentation: Records all procedures and follow-ups.
- Complication Records: Documents any complications with automatic email alerts.
Universal Device Support
EKORA® offer wide compatibility:
- Manufacturer-Agnostic: Supports data collection from all major device manufacturers.
- Wide Compatibility: Works with pacemakers, ICDs, CRTs, loop recorders and non-standard device placements/combinations.
Powerful Search Tools
EKORA® enhances efficiency with its search capabilities:
- Fast Search: Quickly search across all device patients.
- Enhanced Clinical Safety: Reduces clinical risks with accurate data retrieval.
- Multi-Variable Search: Allows searches across multiple variables and exports results.
- Audit and Research Support: Facilitates clinical workflow, audit, and research.
Seamless Reporting Functions
Reporting is straightforward with EKORA®:
- Comprehensive PDF Reports: Standardised reports for implants and follow-ups.
- Email Integration: Sends reports to clinicians and files emails within patient records.
- Audit Data Feeds: Can feed national audit data to Public Health Scotland and UK NICOR.
Enhancing Clinical Workflow
EKORA® aids clinical workflow with several features:
- Calendar View: Manages implants and follow-ups efficiently.
- Group Management: Allows adding patients to multiple groups for easier management.
Unique, Functional Features
EKORA’s Devices module offers additional functionalities not offered by other platforms:
- Alerts and Notices: Adds patient, device, and lead-level alerts.
- Wound Image Upload: Uploads images for follow-up across different sites.
- ID Card Production: Produces ID cards with relevant patient and device/lead details.
- Driving Status Management: Manages driving status and restrictions.
In summary, the EKORA® Devices module provides a comprehensive and efficient solution for cardiology departments. It replaces paper records with a digital system, improving clinical workflow, patient management, and data reporting, making it a valuable tool for cardiologists.
Talk to us today or book a free demo for your department.